Abstract
Introduction: Transformation to secondary myelofibrosis (MF) occurs as part of the natural history of polycythemia vera (PV) and essential thrombocythemia (ET), the two more indolent Ph-negative myeloproliferative neoplasms (MPN). Once transformed, survival is remarkably shorted. Chronic inflammation plays a critical role in the progression of MPN, driving clonal expansion toward end stage disease. Importantly, MPN are characterized by the production of inflammatory cytokines, by both malignant and non-malignant clone. Inflammation and cancer share a common pathway, i.e. NF-κB. Interestingly, miR-146a regulates TLR/NF-κB pathway through the inhibition of its targets, IRAK1 and TRAF6, decreasing the production of cytokines. Based on: i) miR-146a-/- mice develop an MF-like phenotype with aging; and ii) miR-146a polymorphism (miRSNPs) rs2431697, influences its expression levels (50% decrease in TT individuals); we hypothesized that lower miR-146a-5p levels associated to this miRSNPs may result in high risk to develop MF.
Objective: To evaluate the association of rs2431697 with MF transformation and to study the molecular mechanisms beyond this association.
Methods: We genotyped rs2431697 in 938 patients (312 MF, 299 PV, and 327 ET) recruited from 13 tertiary Spanish institutions belonging to GEMFIN and 600 controls. The levels of miR-146a and IRAK1 were evaluated by qRT-PCR in total blood RNA of homozygous patients (TT=30, CC=25) with PV or ET and in healthy subjects (TT=7, CC=7). In miR-146a-/- mice, 2 and 9 months old, we evaluated spleen size and cellularity: degree of fibrosis in bone marrow (H&E and silver staining); and STAT3 and pSTAT3 in granulocytic lysates by western blot.
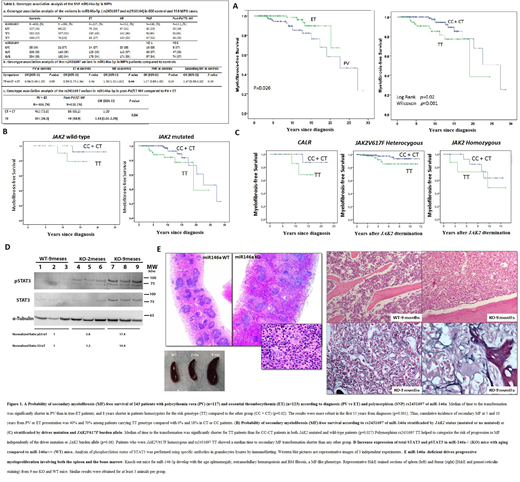
Results: Association analysis, taken controls as reference, showed that TT genotype (associated in the literature with low levels of mir-146a) is associated to MF with an OR of 1.36 (1.01-1.82, p=0.04). Among MF patients, the subgroup with the greatest differences was the one of secondary MF (OR = 1.47, CI: 0.98-2.20) (Table 1 a,b). Next, we compared the genetic frequencies of rs2431697 SNPs between the secondary MF patients and the population in risk. Confirming our hypothesis, we observed an enrichment of TT genotype in the post-PV/TE MF group (n=132) compared to the PV+TE group (n=626) (OR=1.51; p<0.05), Table 1c. In patients with PV or true-ET (WHO criteria) and known clinical follow-up (n=243), excluding pre-fibrotic MF, 8.6% were transformed. The median time to transformation was 27 years, being significantly shorter in patients with PV (vs. ET); homozygous for JAK2V617F; and in TT carriers (vs. TC+CC) (Figure 1A). The mayor differences were seen at early time points (Wilconxon test, p=0.001). In fact, 7 out of 10 TT patients who progress to MF, did so in the first 10 years after diagnosis (70%) as compared with 2 out of 11 CC/CT patients (18%).Both groups (TT and TC+CC) were similar in age, sex, cell counts, initial diagnosis (PV/ET), driver mutations and fibrosis grade 1. In the multivariate analysis, TT genotype remained statistically significative [OR 2.87; CI: 1.19-6.94; p=0.019], independently of phenotype (PV/ET) or V617F allele burden. Both in JAK2 mutated and wild type patients, the time to progression to secondary MF was significantly shorter among TT patients (p=0.027) (Figure 1B). Moreover, TT genotype helped to categorize the risk of progression to MF independently of the driver mutation (JAK2 or CALR) or JAK2 burden allele (p=0.06) (Figure 1C). Consistently, TT patients showed a trends towards a lower expression of miR-146a (p=0.08) and higher IRAK1 (p=0.07) with a significant correlation between both (p<0.01). Finally, we evaluated the association between the JAK-STAT3 and TLR/NF-κB pathways in mice miR-146a-/-. We observed higher total STAT3 and pSTAT3 expression levels in miR-146a-/- than in WT mice (Figure 1D). This increase correlated with aging, and were associated with the appearance of splenomegaly, extramedular hematopoyesis and bone marrow fibrosis at 9 months of age (Figure 1E).
Conclusion: rs2431697-TT is an independent marker of early progression to secondary MF. The lower expression of miR-146a that this SNP confers is associated with an increase in JAK-STAT3 signaling. Our findings include, for the first time, miR-146a in the MPN signaling pathways. Thus, miR-146a, modulating the activation of NF-kB-IRAK1, could indirectly regulates JAK-STAT3 signalling.
CINC424AES05T
Ferrer Marin:Novartis: Consultancy, Research Funding; Incyte: Consultancy. Hernandez Boluda:Incyte: Consultancy; Novartis: Consultancy. García Gutiérrez:Incyte: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Research Funding, Speakers Bureau. Gómez-Casares:Bristol-Myers Squibb: Speakers Bureau; Incyte: Speakers Bureau; Novartis: Speakers Bureau. Besses:Novartis: Honoraria, Research Funding; Shire: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal